Blood Purification and Sepsis Management
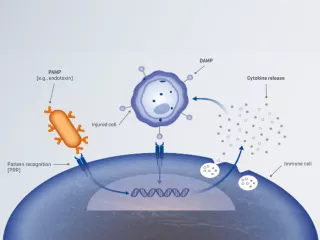
The only therapy that can effectively remove inflammatory mediators, damage-associated molecular patterns (DAMPS) and/or pathogen-associated molecular patterns (PAMPs) (e.g., endotoxin).
Pathogen-associated molecular patterns (PAMPs)
All pathogens exhibit specific components on their surface, known as PAMPs (e.g., endotoxin).1
Damage-associated molecular patterns (DAMPs)
When host cells are damaged, they release endogenous molecules known as DAMPs, such as adenosine triphosphate (ATP), mitochondrial deoxyribonucleic acid (DNA) and high-mobility group box 1 (HMGB1).2,3
Inflammatory mediators, such as cytokines
Inflammatory mediators are key signalling molecules that regulate the inflammatory response, including pro- and anti-inflammatory cytokines, e.g., interleukin (IL)-6, IL-8, IL-10 and tumour necrosis factor (TNF)-α.4
Sepsis and septic shock
- Analysis of data from the Global Burden of Diseases Study estimated the total annual number of sepsis and septic shock cases in 2017 to be 48.9 million, leading to approximately 11 million deaths; this accounted for ~20% of all deaths worldwide.11
- Elevated plasma cytokine levels, particularly IL-6, are associated with an increased risk of mortality following ICU admission in patients with sepsis or septic shock.12-15
- High endotoxin activity is associated with greater illness severity16-18 and organ failure in patients with sepsis/septic shock.16
Multi-organ dysfunction
- Multi-organ failure has been reported in ~75% of critically ill patients with severe sepsis.19
- Combined renal and respiratory failure is common in critically ill patients; ~25% of patients with severe COVID-19 receiving advanced respiratory support also require renal support.20
- Multi-organ dysfunction/failure may be associated with an increased risk of short- and long-term mortality,21-30 contributing to as many as 50% of ICU deaths.21,22
COVID-19
- As of May 2022, the World Health Organization (WHO) reported a total of >513 million confirmed cases of COVID-19 globally, including >6 million deaths.31
- Inflammatory mediators, DAMPs and PAMPs, including endotoxin and SARS-CoV-2 particles, are possible contributors to multi-organ failure in critically ill COVID-19 patients.5
Benefits of blood purification therapy
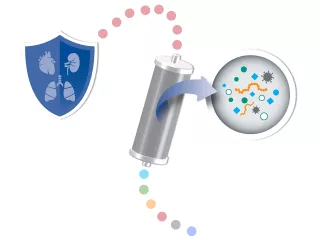
Blood purification is defined as the process of removing mediators and toxins, which may cause pathogenesis, from the blood.1,32
Blood purification therapies are designed to remove inflammatory mediators, PAMPs (such as endotoxin) and/or DAMPs (such as HMGB-1) to address the effects of an unbalanced immune system.1,33
Evidence suggests that the use of blood purification therapies is associated with a decrease in norepinephrine dose/infusion rate in patients with sepsis or septic shock.34-38
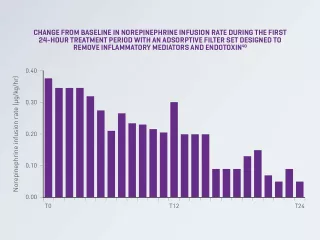
A randomised, crossover, double-blind study of patients with septic shock and AKI reported an 87% decrease in norepinephrine infusion rate from baseline after 24 hours of treatment with continuous renal replacement therapy (CRRT) and blood purification therapy using an adsorptive filter set designed to remove inflammatory mediators and endotoxin (P= 0.02).38
Evidence suggests that the use of blood purification therapies is associated with clinical improvement in organ function (as measured by SOFA score) in patients with severe COVID-19 with or without AKI.39,40
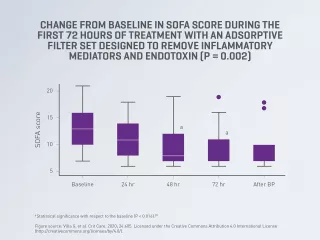
A prospective, multicenter, observational study reported an improvement in organ function, as measured by SOFA score (P = 0.002), in critically ill patients with severe COVID-19 treated with blood purification therapy using an adsorptive filter set removing inflammatory mediators and endotoxin (a PAMP), as well as lower observed mortality vs. predicted mortality as measured by APACHE IV score.39,40
Blood purification has the potential to help mitigate multi-organ failure and improve outcomes.34-42
Blood purification is the only therapy that can effectively remove inflammatory mediators, DAMPS and/or PAMPs (e.g., endotoxin),1 which may help mitigate multi-organ failure and improve patient outcomes.34-42
Blood purification therapy for COVID-19
The connection between COVID-19 and sepsis
In this editorial, Jean-Louis Vincent, MD, PhD, Professor of Intensive Care Medicine at l’Université libre de Bruxelles, discusses how COVID-19 is a dysregulated host response to infection.43
COVID-19 can affect a number of organs and tissues44
- Brain: strokes, seizures, mental confusion and brain inflammation
- Eyes and nose: loss of smell and conjunctivitis
- Lungs: alveoli cell wall destruction, diminishing oxygen uptake; coughing, fever and dyspnea
- Heart and blood vessels: blood clots, myocardial infarction and cardiac inflammation
- Liver: abnormal enzyme levels
- Kidneys: kidney damage that may result from direct viral infection of the kidneys or septic shock
- Intestines: : ≥20% of patients having diarrhoea
Vantive, Oxiris, Prismaflex and PrisMax are trademarks of Vantive Health LLC or its affiliates.
References
-
Monard C, Rimmelé T, Ronco C. Extracorporeal blood purification therapies for sepsis. Blood Purif. 2019;47(suppl 3):1-14.
-
Gotts JE, Matthay MA. Sepsis: pathophysiology and clinical management. BMJ. 2016;353:i1585.
-
Schaefer L. Complexity of danger: the diverse nature of damage-associated molecular patterns. J Biol Chem. 2014;289(51):35237-35245.
-
Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801-810
-
Nadim MK, Forni LG, Mehta RL, et al. COVID-19-associated acute kidney injury: consensus report of the 25th Acute Disease Quality Initiative (ADQI) Workgroup. Nat Rev Nephrol. 2020;16(12):747-764
-
Husain-Syed F, Slutsky AS, Ronco C. Lung-kidney cross-talk in the critically ill patient. Am J Respir Crit Care Med. 2016;194(4):402-414.
-
Shen Y, Cui N, Miao B, et al. Immune dysregulation in patients with severe acute pancreatitis. Inflammation. 2011;34:36-42.
-
Ilonen I, Koivusalo AM, Repo H, Höckerstedt K, Isoniemi H. Cytokine profiles in acute liver failure treated with albumin dialysis. Artif Organs. 2008;32(1):52-60.
-
Shimabukuro-Vornhagen A, Gödel P, Subklewe M, et al. Cytokine release syndrome. J Immunother Cancer. 2018;6:56.
-
Jeschke MG, van Baar ME, Choudhry MA, Chung KK, Gibran NS, Logsetty S. Burn injury. Nat Rev Dis Primers. 2020;6(1):11.
-
Rudd KE, Johnson SC, Agesa KM, et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. Lancet. 2020;395(10219):200-211.
-
Andaluz-Ojeda D, Bobillo F, Iglesias V, et al. A combined score of pro- and anti-inflammatory interleukins improves mortality prediction in severe sepsis. Cytokine. 2012;57(3):332-336.
-
Mat-Nor MB, Md Ralib A, Abdulah NZ, Pickering JW. The diagnostic ability of procalcitonin and interleukin-6 to differentiate infectious from noninfectious systemic inflammatory response syndrome and to predict mortality. J Crit Care. 2016;33:245-251.
-
Barre M, Behnes M, Hamed S, et al. Revisiting the prognostic value of monocyte chemotactic protein 1 and interleukin-6 in the sepsis-3 era. J Crit Care. 2018;43:21-28.
-
Bozza FA, Salluh JI, Japiassu AM, et al. Cytokine profiles as markers of disease severity in sepsis: a multiplex analysis. Crit Care. 2007;11(2):R49.
-
Biagioni E, Venturelli C, Klein DJ, et al. Endotoxin activity levels as a prediction tool for risk of deterioration in patients with sepsis not admitted to the intensive care unit: a pilot observational study. J Crit Care. 2013;28(5):612-617.
-
Ikeda T, Ikeda K, Suda S, et al.Usefulness of the endotoxin activity assay as a biomarker to assess the severity of endotoxemia in critically ill patients. Innate Immun. 2014;20:881-887.
-
Ikeda T, Kamohara H, Suda S, et al. Comparative evaluation of endotoxin activity level and various biomarkers for infection and outcome of icu-admitted patients. Biomedicines. 2019;7:47.
-
Blanco J, Muriel-Bombín A, Sagredo V, et al. Incidence, organ dysfunction and mortality in severe sepsis: a Spanish multicentre study. Crit Care. 2008;12(6):R158. doi:10.1186/cc7157.
-
ICNARC. ICNARC report on COVID-19 in critical care: England, Wales and Northern Ireland 08 April 2022. Accessed April 2022. Available at: https://www.icnarc.org/our-audit/audits/cmp/reports
-
Mayr VD, Dünser MW, Greil V, et al. Causes of death and determinants of outcome in critically ill patients. Crit Care. 2006;10(6):R154.
-
Orban JC, Walrave Y, Mongardon N, et al. Causes and characteristics of death in intensive care units: a prospective multicenter study. Anesthesiology. 2017;126(5):882-889.
-
Lie KC, Lau C, Chau NV, et al. Utility of SOFA score, management and outcomes of sepsis in Southeast Asia: a multinational multicenter prospective observational study. J Int Care. 2018;6:9.
-
Lone NI, Walsh TS.Impact of intensive care unit organ failures on mortality during the five years after a critical illness. Am J Respir Crit Care Med. 2012;186:640-647.
-
Jain A, Palta S, Saroa R, Palta A, Sama S, Gombar S. Sequential organ failure assessment scoring and prediction of patient's outcome in intensive care unit of a tertiary care hospital. J Anaesthesiol Clin Pharmacol. 2016;32(3):364-368.
-
Ferreira FL, Bota DP, Bross A, Mélot C, Vincent JL. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 2001;286(14):1754-1758.
-
Liu J, Zhang S, Wu Z, et al. Clinical outcomes of COVID-19 in Wuhan, China: a large cohort study. Ann Intensive Care. 2020;10:99.
-
Jin M, Lu Z, Zhang X, et al. Clinical characteristics and risk factors of fatal patients with COVID-19: a retrospective cohort study in Wuhan, China. BMC Infect Dis. 2021;21:951.
-
Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-1062.
-
Juvé-Udina ME, Adamuz J, López-Jimenez MM, et al. Predicting patient acuity according to their main problem. J Nurs Manag. 2019;27(8):1845-1858.
-
WHO COVID-19 Dashboard. 2022. Accessed May 2022. Available at: https://www.covid19.who.int.
-
Rimmelé T, Kellum JA. Clinical review: blood purification for sepsis. Crit Care. 2011;15(1):205.
-
Malard B, Lambert C, Kellum JA. In vitro comparison of the adsorption of inflammatory mediators by blood purification devices. Intensive Care Med Exp. 2018;6(1):12.
-
Turani F, Barchetta R, Falco M, Busatti S, Weltertet L. Continuous renal replacement therapy with the adsorbing filter Oxiris in septic patients: a case series. Blood Purif. 2019;47(suppl 3):54-58.
-
Schwindenhammer V, Girardot T, Chaulier K, et al. oXiris® use in septic shock: experience of two french centres. Blood Purif. 2019;47:29-35.
-
Lumlertgul N, Srisawat N. The haemodynamic effects of oXiris haemofilter in septic shock patients requiring renal support: a single-centre experience. Int J Artif Organs. 2020;44:17-24.
-
Hawchar F, László I, Öveges N, Trásy D, Ondrik Z, Molnar Z. Extracorporeal cytokine adsorption in septic shock: a proof of concept randomized, controlled pilot study. J Crit Care. 2019;49:172-178.
-
Broman ME, Hansson F, Vincent JL, Bodelsson M. Endotoxin and cytokine reducing properties of the oXiris membrane in patients with septic shock: a randomized crossover double-blind study. PLoS One. 2019;14:e0220444.
-
Villa G, Romagnoli S, De Rosa S, et al. Blood purification therapy with a hemodiafilter featuring enhanced adsorptive properties for cytokine removal in patients presenting COVID-19: a pilot study. Crit Care. 2020;24:605.
-
Nassiri AA, Hakemi MS, Miri MM, Shahrami R, Koomleh AA, Sabaghian T. Blood purification with CytoSorb in critically ill COVID-19 patients: a case series of 26 patients. Artif Organs. 2021;45(11):1338-1347.
-
Cruz DN, Antonelli M, Fumagalli R, et al. Early use of polymyxin B hemoperfusion in abdominal septic shock: the EUPHAS randomized controlled trial. JAMA. 2009;301(23):2445-52.
-
Rosalia RA, Ugurov P, Neziri D, et al. Extracorporeal blood purification in moderate and severe COVID-19 patients: a prospective cohort study. Blood Purif. 2022;51:233-242.
-
Vincent JL. COVID-19: it's all about sepsis. Future Microbiol. 2021;16:131-133.
-
Wadman M, Couzin-Frankel J, Kaiser J, Matacicet C. A rampage through the body. Science. April 17, 2020. Accessed April 2020. Available at: https://www.science.org/content/article/how-does-coronavirus-kill-clinicians-trace-ferocious-rampage-through-body-brain-toes.