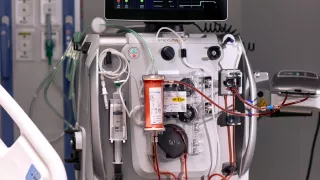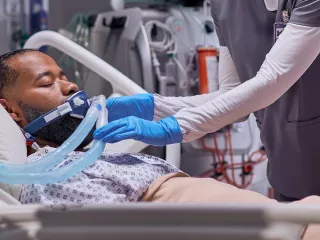
ECCO2R Therapy
Support management of acute respiratory dysfunction with extracorporeal CO2 removal (ECCO2R).
Why ECCO2R therapy?
Acute hypercapnia may be a barrier to the use of guideline-recommended lung-protective ventilation (LPV) and noninvasive ventilation (NIV) strategies. Extracorporeal carbon dioxide removal therapy removes CO2 from a patient’s blood with an extracorporeal circuit to effectively manage acute hypercapnia and respiratory acidosis that can result from lung-protective mechanical ventilation strategies.
Enabling recommended treatment strategies
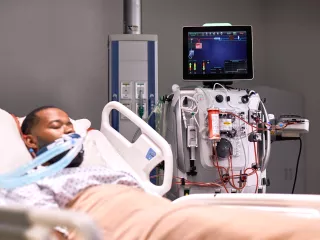
Acute hypercapnia: addressing barriers in caring for your patients
ECCO2R therapy can effectively manage acute hypercapnia to help bring the clinical and economic benefits of LPV and NIV to more patients.
Acute hypercapnia can be a barrier to the use of guideline-recommended LPV and NIV strategies:
- Invasive mechanical ventilation (IMV) with higher plateau pressures, tidal volumes or driving pressures can lead to ventilator-induced lung injury (VILI) and may contribute to multi-organ dysfunction.1-5
- Consequently, clinical guidelines recommend using mechanical ventilation strategies to decrease the risk of lung injury in patients with acute respiratory failure, including LPV,6-8 ultra lung-protective ventilation (ULPV),9 or NIV.10
ARDS: Facilitating use of ventilation strategies for patients with ARDS
- ECCO2R therapy can effectively manage hypercapnia to facilitate the use of LPV or ULPV in patients with mild-to-moderate ARDS.11-14
- ECCO2R therapy may help manage hypercapnia in patients with ARDS secondary to COVID-19.15
- ECCO2R therapy–enabled LPV and ULPV strategies may provide a cost-effective survival benefit in patients with moderate ARDS.16
- In a preliminary analysis using a simulation and comparison of health outcomes and healthcare costs for three IMV strategies in patients with moderate ARDS, survival rates at Day 60 were higher for LPV (63.4%) and ULPV (70.4%) enabled by ECCO2R therapy compared with standard IMV* (57.1%).16
- For lifetime cost-effectiveness outcomes, higher quality-adjusted life years (QALYs) were reported for LPV (2.771) and ULPV (3.203) enabled by ECCO2R therapy compared with standard IMV*(2.595).16 Note, comparisons represent numerical differences as no statistical analyses were conducted.
- In multiway probabilistic sensitivity analyses, ULPV enabled by ECCO2R therapy had the highest probability of cost-effectiveness with a willingness to pay of ≈€22,500 per QALY.16 Note that comparisons represent numerical differences, as no statistical analyses were conducted.
*Standard IMV: tidal volume (VT) ~7–8 mL/kg PBW and plateau pressure (Pplat) ~20–30 cmH2O (as described in the LUNG SAFE study);17 LPV: VT ~6 mL/kg PBW and Pplat ~20–30 cmH2O + ECCO2R initiated for patients with PaCO2 > 55 mmHg during LPV before starting ECCO2R therapy; ULPV: VT ~3–4 mL/kg PBW and Pplat ~20–25 cmH2O + ECCO2R for all patients.16
aeCOPD: Enabling use of NIV strategies for patients with aeCOPD
ECCO2R therapy can effectively manage acute hypercapnia to enable the use of NIV strategies which reduce the need for intubation in patients with aeCOPD.
- ECCO2R therapy, at blood flow ≤ 450 mL/min, may help reduce the need for IMV in patients with aeCOPD.18
- ECCO2R therapy can help to prevent intubation and IMV in patients with aeCOPD at risk of NIV failure.15, 19-21
- Data from a matched cohort study using historic controls indicate that patients with aeCOPD receiving NIV and ECCO2R therapy had a 73% decreased risk of intubation (HR 0.27; 95% CI 0.07–0.98; P = 0.047) compared with patients receiving NIV alone.19
ECCO2R therapy may facilitate weaning from IMV in patients with aeCOPD.15,20,22
A multi-centre, prospective, observational cohort study with no control group reported that 76% of patients receiving IMV for aeCOPD were able to be successfully weaned with support from ECCO2R therapy.15
Multiple organ failure: integrating with other organ support therapies
ECCO2R therapy can be integrated with other organ support therapies for more efficient management of patients with multiple organ dysfunction.
- Patients with acute respiratory failure often experience other forms of organ dysfunction, including renal failure.18,20,23,24
- ECCO2R therapy can be used at blood flow rates of ≤450 mL/min,18 enabling integration with other organ support therapies, such as CRRT or blood purification using the same platform.18,23
- Clinicians can provide multiple organ support therapies using a single vascular access, helping to minimise the invasiveness of treatment18,20,23 and reduce the risk of infection for patients with multiple organ dysfunction.25-27
… respiratory diseases are a leading cause of death and disability in the world ….” “prevention, control, and cure of these diseases and … promotion of respiratory health must be a top priority for health-care systems and decision makers.28
Forum of International Respiratory Societies
Understanding COVID-19 and ECCO2R therapy
ECCO2R therapy can support the use of LPV by managing elevated CO2 levels:
- Respiratory cells can become infected with the SARS-CoV-2 virus.29
- Viral infection and a dysregulated inflammatory immune response can both contribute to lung injury.30,31
- Lung injury can present as type L pneumonia, which can progress to severe “ARDS-like” type H, characterised by oedema, tissue damage and severe hypoxemia.32,33
- The complications of severe lung injury and its treatment can lead to multiorgan dysfunction, including acute kidney injury.31,34
Vantive, PrismaLung+ and PrisMax are trademarks of Vantive Health LLC or its affiliates.
References
-
Slutsky AS. History of mechanical ventilation. From vesalius to ventilator-induced lung injury. Am J Respir Crit Care Med. 2015;191(10):1106-15.
-
Gattinoni L Protti A.Guidelines for the management of chronic kidney disease. CMAJ. 2008;178:1174-1176.
-
Dreyfuss D, Saumon G. Ventilator-induced lung injury: Lessons from experimental studies. Am J Respir Crit Care Med. 1998;157(1):294-323.
-
Tremblay L, Valenza F, Ribeiro SP, Li J, Slutsky AS. Injurious ventilatory strategies increase cytokines and c-fos m-RNA expression in an isolated rat lung model. J Clin Invest. 1997;99(5):944-52.
-
Brodie D, Slutsky AS, Combes A. Extracorporeal life support for adults with respiratory failure and related indications: a review. JAMA. 2019;322(6):557-568.
-
Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med. 2017;45(3):486-552.
-
ARDSNet. NIH NHLBI ARDS Clinical Network Mechanical Ventilation Protocol Summary. Accessed August 2021. Available at: http://www.ardsnet.org/files/ventilator_protocol_2008-07.pdf.
-
Papazian L, Aubron C, Brochard L, et al. Formal guidelines: management of acute respiratory distress syndrome. Ann Intensive Care. 2019;9(1):69.
-
Alhazzani W, Evans L, Alshamsi F, et al. Surviving sepsis campaign guidelines on the management of adults with coronavirus disease 2019 (COVID-19) in the ICU: First update. Crit Care Med. 2021;49(3):e219-e234.
-
GOLD 2021 Report. 2021. Accessed August 2021. Available at: https://goldcopd.org/wp-content/uploads/2020/11/GOLD-REPORT-2021-v1.1-25Nov20_WMV.pdf.
-
Fanelli V, Ranieri MV, Mancebo J, et al. Feasibility and safety of low-flow extracorporeal carbon dioxide removal to facilitate ultra-protective ventilation in patients with moderate acute respiratory distress sindrome. Crit Care. 2016;20:36.
-
Schmidt M, Jaber S, Zogheib E, Godet T, Capellier G, Combes A. Feasibility and safety of low-flow extracorporeal CO2 removal managed with a renal replacement platform to enhance lung-protective ventilation of patients with mild-to-moderate ARDS. Crit Care. 2018;22(1):122.
-
Winiszewski H, Aptel F, Belon F, et al. Daily use of extracorporeal CO2 removal in a critical care unit: indications and results. J Intensive Care. 2018;6:36.
-
Combes A, Fanelli V, Pham T, et al. Feasibility and safety of extracorporeal CO2 removal to enhance protective ventilation in acute respiratory distress syndrome: the SUPERNOVA study. Intensive Care Med. 2019;45(5):592-600.
-
Augy J, Aissaoui N, Richard C. A 2-year multicenter, observational, prospective, cohort study on extracorporeal CO2 removal in a large metropolis area. J Intensive Care. 2019;7:45.
-
Ethgen O, Goldstein J, Harenski K, et al. A preliminary cost-effectiveness analysis of lung protective ventilation with extra corporeal carbon dioxide removal (ECCO2R) in the management of acute respiratory distress syndrome (ARDS). J Crit Care. 2021;63:45-53.
-
Bellani G, Laffey JG, Pham T, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016 Feb 23;315(8):788-800.
-
Allardet-Servent J, Castanier M, Seignouret T, Soundaravelou R, Lepidi A, Seghboyan JM. Safety and efficacy of combined extracorporeal CO2 removal and renal replacement therapy in patients with acute respiratory distress syndrome and acute kidney injury: the pulmonary and renal support in acute respiratory distress syndrome study. Crit Care Med. 2015;43(12):2570-2581.
-
Del Sorbo L, Pisani L, Filippini C, et al. Extracorporeal CO2 removal in hypercapnic patients at risk of noninvasive ventilation failure: a matched cohort study with historical control. Crit Care Med. 2015;43(1):120-127.
-
Consales G, Zamidei L, Turani F, et al. Combined renal-pulmonary extracorporeal support with low blood flow techniques: a retrospective observational study (CICERO Study). Blood Purif. 2022;51(4):299-308.
-
Kluge S, Braune SA, Engel M, et al. Avoiding invasive mechanical ventilation by extracorporeal carbon dioxide removal in patients failing noninvasive ventilation. Intensive Care Med. 2012;38(10):1632-1639.
-
Abrams D, Brodie D. Emerging indications for extracorporeal membrane oxygenation in adults with respiratory failure. Ann Am Thorac Soc. 2013;10(4):371-377.
-
Nentwich J, Wichmann D, Kluge S, Lindau S, Mutlak H, John S. Low-flow CO2 removal in combination with renal replacement therapy effectively reduces ventilation requirements in hypercapnic patients: a pilot study. Ann Intensive Care. 2019;9(1):3.
-
ICNARC. ICNARC report on COVID-19 in critical care: England, Wales and Northern Ireland. 13 August 2021. Accessed August 2021. Available at: https://www.icnarc.org/our-audit/audits/cmp/reports.
-
Joint Commission. Central line-associated bloodstream infections toolkit and monograph. Accessed August 2021. Available at: https://www.jointcommission.org/-/media/tjc/documents/resources/hai/clabsi_monographpdf.pdf.
-
Dube WC, Jacob JT, Zheng Z. Comparison of rates of central line-associated bloodstream infections in patients with 1 vs 2 central venous catheters. JAMA Netw Open. 2020;3(3):e200396.
-
Concannon C, van Wijngaarden E, Stevens V, Dumyati G. The effect of multiple concurrent central venous catheters on central line–associated bloodstream infections. Infect Control Hosp Epidemiol. 2014;35(9):1140-1146.
-
Forum of International Respiratory Societies. The Global Impact of Respiratory Disease. 2nd Edition. 2017.
-
Albini A, Di Guardo G, Noonan DM, et al. The SARS-CoV-2 receptor, ACE-2, is expressed on many different cell types: implications for ACE-inhibitor- and angiotensin II receptor blocker-based cardiovascular therapies. Intern Emerg Med. 2020;15:759-766.
-
Li G, Fan Y, Lai Y, et al. Coronavirus infections and immune responses. J Med Virol. 2020;92(4):424-432.
-
Batlle D, Soler MJ, Sparks MA, et al. Acute kidney injury in COVID-19: emerging evidence of a distinct pathophysiology. J Am Soc Nephrol. 2020;31(7):1380-1383.
-
Gattinoni L, Chiumello D, Caironi P, et al. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020;46(6):1099-1102.
-
Gattinoni L, Chiumello D, Rossi S. COVID-19 pneumonia: ARDS or not? Crit Care. 2020;24(1):154.
-
Ronco C, Reis T. Kidney involvement in COVID-19 and rationale for extracorporeal therapies. Nat Rev Nephrol. 2020;16(6):308-310.